The role of sleep in emotional brain function - inquiry essay article analysis (week 3)
*Note: this is only a section of the whole article
Impact of sleep loss on emotional brain function: Reactivity and recognition
Together with impairments of attention, alertness and memory, sleep loss has consistently been associated with subjective reports of irritability and emotional volatility (Horne 1985). Restricting sleep to only 5hr a night across a 1-week period leads to a progressive increase in emotional disturbance in participants on the basis of questionnaire mood scales, together with diary documentation of increasing subjective emotional difficulties (Dinges et al 1997). Moreover, accumulated sleep loss leads to an amplification of negative emotions in response to disruptive daytime experiences, while blunting the affective benefit associated with goal-enhancing activities (Zohar et al 2005). Congruently, one night of experimentally controlled sleep loss increases subjective reports of stress, anxiety and anger in response to low-stress situations (Minkel et al 2012), and increases impulsivity towards negative stimuli (Anderson & Platten 2011). This is of particular clinical interest considering that impulsivity is significantly correlated with aggressive behavior and suicidality (Plutchik 1995), both of which are associated with sleep disruption (Bernert & Joiner 2007, Kamphuis et al 2012).
Studies assessing objective physiological and neural measures of affect have provided additional verification of, and explanatory mechanisms for, emotional dysregulation following sleep deprivation. Assessed using functional MRI (fMRI), one night of sleep deprivation triggers a 60% amplification in reactivity of the amygdala in response to emotionally negative pictures, relative to a normal night of sleep (Figure 1a&b) (Yoo et al 2007). Moreover, this increase in amygdala reactivity is paired with a reduction in functional connectivity with regions of the mPFC that exert top-down regulatory control of the amygdala (Figure 1c&d), yet increased coupling with the fight/flight adrenergic-activating brainstem center of the locus coeruleus. A similar profile of exaggerated amygdala reactivity and reduced prefrontal connectivity occurs after 5 nights of 4hr sleep restriction; arguably a more ecologically relevant paradigm in the context of societal sleep behavior and clinical disorders (Motomura et al 2013). Moreover, habitual subjective sleep quality outside of the laboratory is also significantly related to amygdala reactivity, as well as features of negative affect and stress (Prather et al 2013). Additionally, inter-individual differences in the change in connectivity between the amygdala and mPFC caused by sleep deprivation accurately predicts the concurrent increase in subjective anxiety (Motomura et al 2013).
Figure 1.
The impact of sleep deprivation on emotional brain reactivity and functional connectivity.
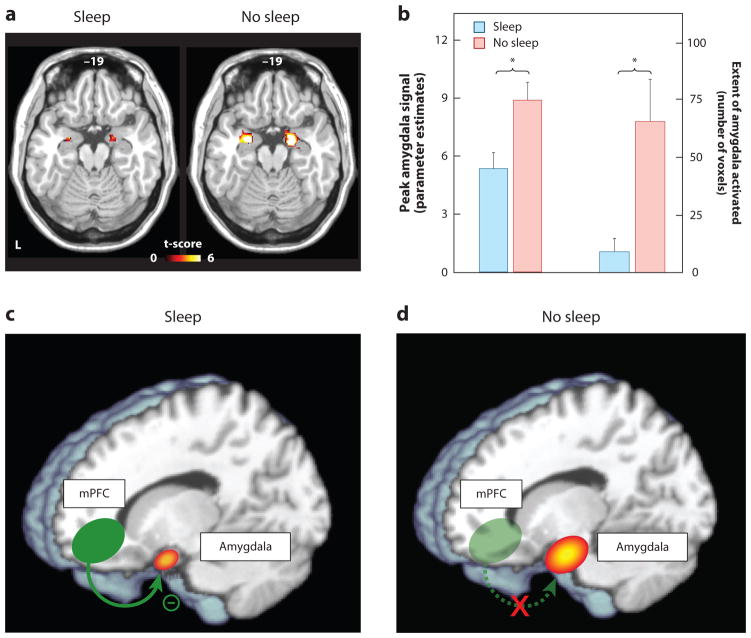
(a) Amygdala response to increasingly negative emotional stimuli in the Sleep deprivation and Sleep rested (control) conditions, and (b) Corresponding differences in intensity and volumetric extent of amygdala activation between the two groups (average ± s.e.m. of left and right amygdala), (c) Changes in functional connectivity between the medial prefrontal cortex (mPFC) and the amygdala.
With sleep, the prefrontal lobe was significantly connected to the amygdala, regulating and exerting and inhibitory top-down control, (d) Without sleep, however, amygdala-mPFC connectivity was decreased, potentially negating top-down control and resulting in an overactive amygdala. *p < 0.01; error bars indicate s.e.m.
Modified from (Yoo et al 2007).
While the majority of studies to date have focused on changes within the central nervous system (and specifically brain), it is of note that such central alterations are paralleled by changes in peripheral nervous system physiology. One night of sleep deprivation amplifies pupil diameter responses—an index of peripheral autonomic nervous system reactivity—during the passive viewing of negative emotional picture stimuli (Franzen & Buysse 2008). Congruently, sleep deprivation also increases sympathetic dominance of the autonomic nervous system, indexed by changes in heart rate variability (Sauvet et al 2010, Zhong et al 2005). The latter is important, since this sympathetic bias is associated with a lack of flexibility and capacity to respond to emotional challenges, and has been positively associated with psychopathology (Appelhans 2006).
Growing evidence suggests that sleep loss imposes a bi-directional nature of affective imbalance, additionally triggering excessive reactivity to positive, reward-relevant stimuli. One night of sleep loss enhances reactivity throughout regions of the dopaminergic mesolimbic systems in response to pleasure-evoking emotional picture stimuli (Gujar et al 2011). As with aversive reactivity, this enhanced mesolimbic reward sensitivity is further associated with decreased functional connectivity in regions of the medial and orbital prefrontal cortex. Similar enhanced mesolimbic reactivity following sleep deprivation can occur using monetary reward incentive paradigms (Libedinsky et al 2011, McKenna et al 2007, Venkatraman et al 2007). Beyond more abstract reward stimuli, such as money, this impact of sleep loss extends to more primary reward-motivated behaviors – that of appetitive food desire. Both acute and chronic sleep loss are associated with elevated reactivity to food stimuli in salience and hedonic regions of the striatum and amygdala, together with blunted activity in appetitive choice and decision making regions of the frontal cortex (Benedict et al 2012, Greer et al 2013, Killgore et al 2013, St-Onge et al 2012). Moreover, these neural changes are accompanied by an increased preference for higher calorie foods (Greer et al 2013) and greater tendencies to overeat (Killgore et al 2013).
In addition to changes in neural reactivity at the time of experiencing an emotional event, sleep loss further alters the preemptive neural anticipation of impending emotional experiences in both the amygdala and anterior insula cortex (Goldstein et al 2013). Of special clinical relevance, trait anxiety levels predict inter-individual differences in the extent of this exaggerated sleep deprived anticipation response (Goldstein et al 2013). Specifically, high trait anxious participants express the most severe increase in anticipatory reactivity under conditions of sleep loss; concerning considering these are already the individuals at greatest risk for developing an anxiety disorder.
Amplified anticipatory responding following sleep deprivation is also observed for reward-relevant cues. One night of sleep deprivation elevates anticipatory activity within the reward-sensitive region of the striatum to monetary decisions that can lead to either future reward payoffs or losses – especially when those gambles are risky (Venkatraman et al 2007). Consistent with studies discussed earlier regarding appetitive food stimuli, the increases in subcortical striatal reactivity following sleep deprivation co-occurs with blunted activity in anterior insula cortex and orbitofrontal cortex, specifically to monetary losses. Thus, sleep deprivation appears to trigger a state were rewards are overvalued (increased striatal sensitivity), yet losses (through punishment) are undervalued.
Taken as a whole, these data establish that insufficient sleep exaggerates subcortical limbic and striatal responses to both negative and positive affective stimuli, commonly associated with impoverished prefrontal cortex activity and/or connectivity. The consequence appears to be a pendulum like, bi-directional state of emotion imbalance at both ends of the valence spectrum, fitting early anecdotal reports of affective liability following a lack of sleep (Dahl 1996). Furthermore, this exaggerated reactivity can if cued, be observed preemptively, before the emotional stimulus itself.
Such a model of altered emotion reactivity following sleep loss is of translational relevance for at least three clinical areas. First, remarkably similar patterns of altered mesolimbic system emotion reactivity, as well as limbic-prefrontal cortex connectivity, have been reported in several affective psychopathologies, including major depression, bipolar disorder, generalized anxiety disorder and PTSD (Davidson 2002, Drevets et al 2008, Etkin 2010, Etkin & Wager 2007, Nitschke et al 2009, Paulus & Stein 2006, Rauch et al 2000, Shin et al 2006, Siegle et al 2007). Crucially, every one of these clinical conditions expresses highly comorbid sleep disruption (Harvey 2011, Peterson & Benca 2006), and in some of these disorders, sleep abnormalities form part of their diagnostic criteria. Considering the overlap between the neural correlates of such conditions that demonstrate co-occurring sleep disruption, and the patterns of neural dysfunction that can be experimentally induced by sleep deprivation, it raises the important issue of whether sleep loss plays a causal role in the etiology of these conditions. Moreover, should sleep be a contributing factor, it would represent a novel treatment intervention target (Harvey et al 2011).
Second, in the context of reward sensitivity, sleep disturbance is a recognized hallmark of addiction (Arnedt et al 2007, Brower & Perron 2010, Dimsdale et al 2007, Pace-Schott et al 2005), leading to the recent proposal that sleep loss represents a common and reliable predictor of relapse in numerous addiction disorders (Brower & Perron 2010). Additionally, a prospective study has demonstrated that sleep problems assessed during childhood significantly predict early onset of drug and alcohol use years later in adolescence, even when controlling for effects of anxiety and attention deficits (Volkow et al 2009). As such, the mesolimbic dopaminergic system appears to represent one common pathway through which the effects of sleep loss and heightened addiction sensitivity can be understood. The experimental evidence discussed earlier, demonstrating an interaction between a lack of sleep and enhanced mesolimbic reward-reactivity, implicates sleep loss as a predisposing and causal (rather than co-occurring) risk factor in heightened responsivity and hence acquired addiction potential to reward-stimulating drugs. Moreover, beyond acquisition, they further indicate a possible role for sleep disruption in the maintenance of addiction habits, especially during attempted withdrawal, leading to higher relapse rates.
Third, while anticipation is generally an adaptive process, aiding preparatory responses to potentially threatening or rewarding events, exaggerated expectancy activity, such as that observed following sleep deprivation, can be maladaptive. In the context of aversive events, increased anticipatory limbic activity positively predicts clinical features of anxiety disorders, such as worry and rumination (Etkin & Wager 2007, Nitschke et al 2009, Paulus & Stein 2006), many of which express co-occurring impairments in the quantity and quality of sleep (Papadimitriou & Linkowski 2005). Perhaps more concerning is that individuals with higher levels of trait anxiety and are therefore already at higher risk for developing an anxiety disorder, appear to be the most vulnerable to these anxiogenic effects of insufficient sleep (Goldstein et al 2013).
Emotion recognition and expression
Intriguingly, a number of studies have reported what may at first be considered a paradoxical blunting, rather than over-estimation, in the subjective rating of emotions in others by sleep-deprived participants. For example, sleep deprivation decreases the subjective intensity ratings of threat-relevant (angry) and reward-relevant (happy) static facial expressions (van der Helm et al 2010). Sleep loss additionally decreases the outward expression of emotion by sleep-deprived individual themselves, as judged by expert raters (Minkel et al 2011). Similarly, decreases in the vocal expression of positive emotion by deprived participants is observed after a single night of sleep loss, suggesting that multiple routes of emotional expression (e.g. facial muscles, vocalizations) are compromised by insufficient sleep (McGlinchey et al 2011). In addition to diminishing outward emotive expression, sleep deprivation also slows the generation of facial reactions in response to visual presentation of faces (Schwarz et al 2013). Of concern, insufficient sleep appears to trigger as much, if not more, of an impact on emotional expression in young children. Recent evidence demonstrates that three-year-olds who do not obtain an afternoon nap show dysregulation of both positive and negative emotional expression in response to emotional stimuli, relative to those who have obtained a nap (Berger et al 2012).
The potential disparity between these impairments and the objective neural data described earlier, which have reported amplifications (rather than impairments) in limbic brain reactivity following sleep deprivation can be reconciled when considering the concomitant neural impairments in the prefrontal cortex. Not only are prefrontal regions implicated in top-down regulatory control of subcortical limbic networks, they critically integrate primary affective signals arising from these subcortical systems (such as the brainstem, limbic system and basal ganglia) into second-order maps of the internal state of the organism (Craig 2010, Craig 2011, Critchley 2005, Critchley 2009, Harrison et al 2010). It has been argued that only through such mapping and hence appreciation of the current state of the body in the frontal lobe, can the brain select appropriate behavioural actions for the organism (actions that include emotion expression) (Craig 2010, Craig 2011, Critchley 2005, Critchley 2009, Harrison et al 2010). Set against this evidence, the above disparate findings may be resolved. Specifically, the sleep-deprived brain may suffer a mismatch between excessive subcortical reactivity yet impaired higher-order prefrontal functioning, the latter preventing optimal integration and hence use of the former, as well as control over the former. As a consequence, there can be a failure of affectively guided judgments, decisions and, down-stream, behavioural emotive (re)actions.





 on the uploaded document.
on the uploaded document.
0 General Document comments
0 Sentence and Paragraph comments
0 Image and Video comments
Sleep deprivation leads to increased reactivity to all stimuli, according to this article. The brain can be stimulated by both positive and negative things and have overactive reactions. This goes to show that sleep deprivation lessens the brain’s ability to control emotions and reactions to emotions, which allows a person to be vulnerable during conversations.
New Conversation
Hide Full Comment
This is actually really interesting, even though it’s not directly related to my question. Because of sleep deprivation, the brain has an increased reward sensitivity, which in some cases can lead to relapse for drugs or alcohol.
New Conversation
Hide Full Comment
General Document Comments 0


This article talks about the science of how sleep deprivation affects how we process emotions. Overall, sleep deprivation usually leads to negative emotional brain function and can result in many emotional disorders such as anxiety, depression, etc. However, I still believe this can provide evidence to the question because the article mentions how sleep deprivation overall lowers the ability to control emotions.
New Conversation
Hide Full Comment